History
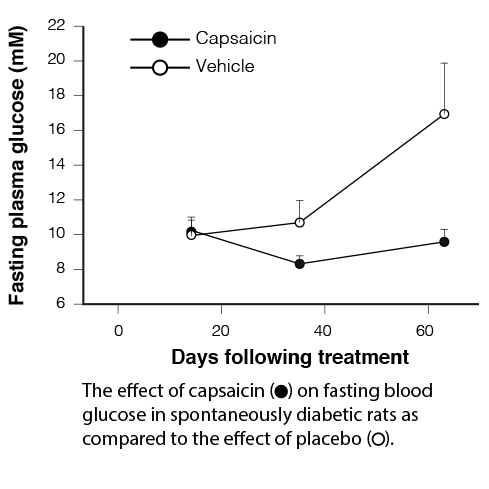
Pila Pharma was founded by Dorte X. Gram after she discovered that TRPV1 antagonists can be used for treatment of diabetes and obesity. These surprising discoveries were made at the turn of the millennium when Dorte X. Gram during her PhD studies, and as part of her work as a researcher at Novo Nordisk A/S, investigated the effects of capsaicin receptor modulator (TRPV1) in animal models. Two decades later, several important milestones have been reached.
Important milestones
| Year | Milestone |
| 1999 | Dorte X. Gram discovers that TRPV1 can regulate blood sugar in diabetic rats through improved insulin secretion |
| 1999-2005 | The Gram-hypothesis is formulated and preclinical studies support the hypothesis |
| 2005 | Dorte X. Gram files use patents on the treatment of diabetes and obesity with TRPV1 antagonists for Novo Nordisk |
| 2008 | For strategic reasons, Novo Nordisk shuts down or sells all projects and patents regarding small molecules
The rights/use patent application on the discovery for treatment of diabetes and obesity with TRPV1 antagonists are acquired by Gram through her company XENIA PHARMA, Denmark, from Novo Nordisk, Denmark |
| 2011 | For strategic reasons, Bayer closes all urogenital projects and patents, and sells its TRPV1 assets to Ario Pharma
Use patent in the US issued to XENIA PHARMA for treatment of obesity with TRPV1 antagonists |
| 2013 | Use patent in the US and Europe issued to XENIA PHARMA for treatment of type 1 and 2 diabetes, insulin resistance and impaired glucose tolerance with TRPV1 antagonists |
| 2014 | Pila Pharma founded in Sweden as a fully owned subsidiary to XENIA PHARMA, Denmark
Use patents are transferred to the Company |
| 2015 | Almi Invest invests and finances preclinical screenings of selected clinical development candidates |
| 2016 | TRPV1 antagonist assets including XEN-D0501 licensed from Ario Pharma, UK (indirectly Bayer, Germany) |
| 2017 | Permission to try single ascending dose for patients with type 2 diabetes with XEN-D0501 (clinical study PP-CT01) |
| 2018 | The concession agreement regarding Ario Pharma TRPV1 renegotiated
The clinical study PP-CT01 shows a good safety profile of XEN-D0501 after patients with type-2 diabetes are treated with single doses Permission to treat patients with type 2 diabetes with XEN-D0501 for 28 days (clinical study PP-CT02) |
| 2019 | The clinical study PP-CT02 is conducted |
| 2020 | Licensing agreement regarding royalties for XEN-D0501 to Ario Pharma ceases
The clinical study PP-CT02 shows a good safety profile and effect of XEN-D0501 on insulin secretion after 28 days of treatment of patients with type-2 diabetes The company’s Board decides on a listing on the stock exchange and Göteborg Corporate Finance is hired as a financial advisor |
| 2021 | During the first quarter, the Company carries out a new share issue, which is oversubscribed The Company receives MSEK 14.1, after issue costs (before listing)
The Company is registered as a public company The Company implements a 10:1 split, with the condition that each share held yields ten new shares During the second quarter, the Company carries out a new share issue in connection with listing on the Nasdaq First North Growth Market in Stockholm, and the Company receives MSEK 31.5, after issue costs Several important agreements are signed with partners, including the British Quay Pharma for manufacture of new tablets, the British Almac for manufacture of API, and the French ERBC for implementation of a three-month preclinical study |
| 2022 | Production of study drug (XEN-D0501 API) was completed with very good results and certificate of analysis obtained
An agreement was signed with British Quay Pharma on the development of a suitable formulation of XEN-D0501 API for use in the upcoming preclinical studies in diabetes type-2 An agreement was signed with British LGC Drug Development Solutions on the establishment of an analytical method to measure XEN-D0501 in samples from the preclinical studies The company held an annual general meeting on June 7, 2022, whereby the board was authorized to, in the time until the next annual general meeting, decide on a new issue of shares, warrants and/or convertibles. At the annual general meeting, Milan Zdravkovic was elected as a board member In July 2022, Pila Pharma was granted Orphan Drug Designation in the USA for XEN-D0501 for the treatment of the rare disease erythromelalgia Dr. Hans Quiding was hired as project manager for work on new projects, including the erythroemalgia project Pila Pharma, in collaboration with ERBC, France, in the period June 2022 to March 2023 conducted a 13-week preclinical safety studies with the development candidate XEN-D0501 in two animal species without registration of side effects, which enables XEN-D0501 to be included in clinical studies of up to 3 months duration During quarter 4, the company carried out a rights issue and the company received SEK 6.9 million after issue costs |
Download as PDF:
use-patent application
Download patents as PDF:
US7879866B2 / US8455504B2 / EP1771162B1