Business model & Strategy
This section is from the Year-End Report for 2023 published on February 28th 2024, and thus changes to the information can have occurred since.
The company’s long-term goal is to register XEN-D0501 as the first TRPV1 antagonist drug.
The company’s short-term goal is to demonstrate the effect of XEN-D0501 on the reduction of blood sugar in type-2 diabetes as well as on the reduction of pain in people affected by erythromelalgia.
“Pila” means “to run fast” and the idea behind the choice of this name was that we should work quickly and cost-effective with a focus on the most essential goals – to focus on “need to do” and avoid “nice to do” as an organizational philosophy.
The company’s intention is to develop the drug candidate XEN-D0501 until a pharma partnership is possible. We focus on consolidating the uniquely good safety profile of the candidate in parallel to gradually add evidence for a clinically meaningful effect in both diabetes (reduction of high blood glucose levels (HbA1c), body weight and risk of cardio-vascular disease) and erythromelalgia (reduction of pain during “flare ups”).
XEN-D0501 is currently formulated as a simple, small tablet with very good shelf life (up to 5 years at 25 C). There is, however, the possibility of developing new formulations for new indications in order to differentiate between the different upcoming drugs for different diseases.
Organizationally, the strategy is to hire experienced specialists to secure the best development methods for different indications. During the period Richard Busellato and Søren Weis Dahl became new Directors of the Board. Richard was invited to join due to his extensive background in the financial industry and deep understanding of capital markets and Søren was invited to join due to his significant expertise in the orphan drug field. In addition, they both add international experience Richard working out of London, UK and Søren out of New York, US. They will both contribute significantly to the further development of the Pila Pharma’s TRPV1 asset.
In addition, Pila Pharma works with a solid core of permanent consultants as well as a number of more peripheral specialist consultants and contract research organizations. This virtual company structure has been further developed during the period and is both strong and flexible and quickly adaptable to changing priorities without losing quality. Quality is essential in drug development, but flexibility is, as we see it, a necessity in order to manage cost-effectively through this long development process
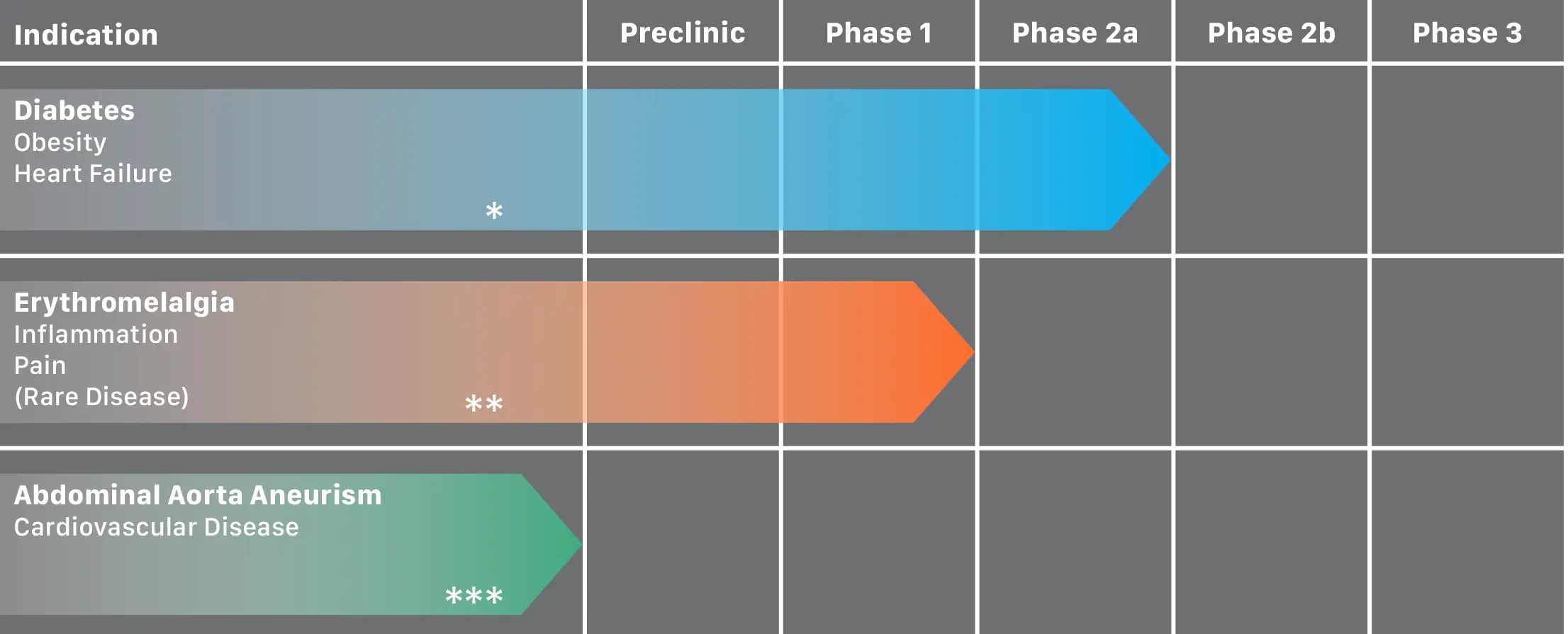
FIG.:
Pila Pharma currently has a pipeline with 3 projects each evaluating the effect of XEN-D0501 in various indications.
* Diabetes Obesity Heart Failure is our primary project and a phase 2a trial is our next step in diabetes/obesity to first identify maximum tolerable dose before progressing to phase 2b – preparations for a clinical trial application is ongoing.
* * Erythromelalgia Inflammation Pain (Rare Disease) is our secondary project but on hold until further funding is secured to conduct a phase 2a testing XEN-D0501 for its effect on pain in persons with Erythromelalgia.
* * * Abdominal Aorta Aneurism /Cardiovascular Disease is a new research collaboration in early preclinical phase where we aim at studying the effect of XEN-D0501 in mice on Abdominal Aorta Aneurism growth.

